Aim of CARE-UK study
In this study, we are recruiting children with asthma and comparing the use of an anti-inflammatory reliever (AIR) inhaler (Symbicort 100/3 MDI) with salbutamol as a reliever. The Symbicort MDI will either be used as needed for children currently prescribed only salbutamol as needed or low dose inhaled corticosteroids (ICS) or as part of Maintenance and Reliever Therapy (MART) for children currently prescribed medium to high dose ICS. We will compare the effectiveness of this approach in terms of asthma attacks, symptom control and quality of life.
Please select which arm your patient is in to expand further details
Intervention arm
The study drug (Symbicort) will be provided by the study team for the duration of the trial (up to 12 months). Your patient should no longer be prescribed salbutamol or their regular ICS or combination inhaler (ICS/LABA). If they are usually prescribed other medication for their asthma or allergies (such as montelukast, antihistamines or nasal sprays) these should be continued.
Any changes in treatment should continue to be managed by the child’s usual healthcare providers. If you feel that their treatment needs to be stepped up or down this should be done in accordance with the steps below. We would ask that you contact the study team if any changes are made, and we can ensure the child has the correct Personal Asthma Action Plan.
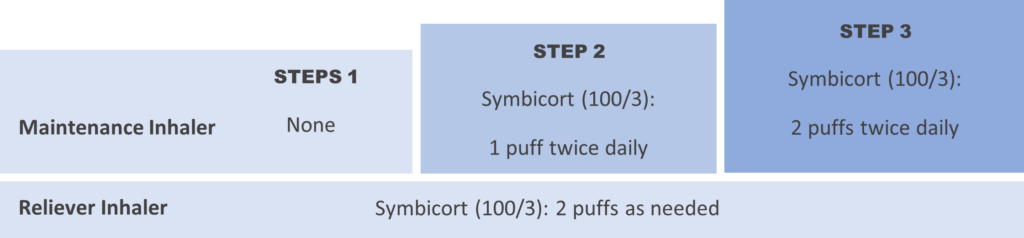
There are three treatment steps.
Step 1: Symbicort (100/3) 2 puffs as needed when symptomatic up to a maximum of 16 puffs per day
Step 2: Symbicort (100/3) 1 puff twice daily for maintenance treatment plus 2 puffs as needed when symptomatic up to a maximum of 16 puffs per day (maintenance and reliever doses)
Step 3: Symbicort (100/3) 2 puffs twice daily for maintenance treatment pus 2 puffs as needed when symptomatic up to a maximum of 16 puffs per day (maintenance and reliever doses)
Control arm
No changes have been made to your patient’s current asthma treatment and any changes in treatment and asthma attacks should continue to be managed as per standard care by their usual healthcare providers.
We will keep you informed of your patient’s progress in the study. We will also keep you, your patient and their parents informed of any new relevant information as it becomes available.
If you have any questions, please contact the chief investigator Dr Louise Fleming via nhs.net.


